What is a health supplement?
Health Supplements are those that are added to the daily diet to maintain, enhance, and improve the functions of the human body, reducing the risk of diseases. These products contain one or more of the following substances:
a) Vitamins, minerals, amino acids, fatty acids, enzymes, probiotics, and other biologically active substances;
b) Naturally sourced substances, including those from animals, minerals, and plants in the form of extracts, isolates, concentrates, and transformations;
c) Health Supplements are formulated in the form of soft/hard capsules, tablets, film-coated/sugar-coated tablets, granules, powder, liquid, and other formulations, and are dosed into small units, depending on the formulation of the product.
According to the provisions of Clause 1, Article 6 of Decree 15/2018/ND-CP, organizations and individuals trading in health supplements must register the product declaration with the Ministry of Health (Department of Food Safety) before putting them into circulation on the market.
Some requirements for the content of health supplement declaration
An entity that makes a product declaration must comply with the requirements specified in Circular 43/2014/TT-BYT on the management of functional foods. Accordingly, the dossier for declaring a health supplement is prepared based on the general requirements for functional foods and the requirements for health supplements.
Some requirements for the content of the declaration such as:
- The declaration of content
- Health recommendations and users
- Requirements for Vietnamese labeling
Documents for registering the product declaration of health supplement
- Product announcement document
- Certificate of compliance with Good Manufacturing Practice (GMP)
- Product testing Results (valid for 12 months)
- Documentation proving the product’s functions or the functions of its components that have been announced
- Dosage table, Recommended Nutrient Intake (RNI) table
- Product Specification (SPEC)
- Product label sample
- Certificate of free sale (CFS) – Requires consular legalization and Expected supplementary label content (for imported products)
 A sample of product declaration of health supplement
A sample of product declaration of health supplement
Instructions for registering the declaration of health supplement
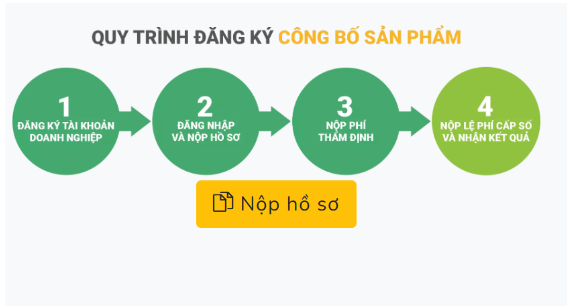
Step 1: Prepare documents and submit the application
Individuals and organizations can submit the registration dossier of the declaration of health supplement to the Ministry of Health (Department of Food Safety) in the following 3 ways:
– Through the online public service system
– By post
– Submit directly
Step 2: Appraisal of documents
The time for appraisal of documents is calculated from the time the documents are submitted on the online public service system or according to the stamp of the receiving agency (in case of submitting documents by post or submitting documents directly).
Step 3: Issuance of a certificate of registration of health supplement
Within 21 working days, the Food Safety Department is responsible for reviewing the dossier and issuing a Certificate of registration of product declaration to the registering individual or organization.
Note: In case the product has a change in product name, origin, or composition, the organization or individual must re-declare the product.
In case of other changes, a written notice of the change must be sent to the dossier receiving agency of the Food Safety Department according to regulations.
The Food Safety Department is responsible for publicly announcing the name and product of the organization or individual that has been accepted for registration of the product declaration on its electronic information page (website) and the food safety database.
Product declaration registration process via the online public service system


 Tiếng Việt
Tiếng Việt